Updated January 2023 By Dr Jonathan Palmer
The Ultimate Guide To Hair pH
What is pH?
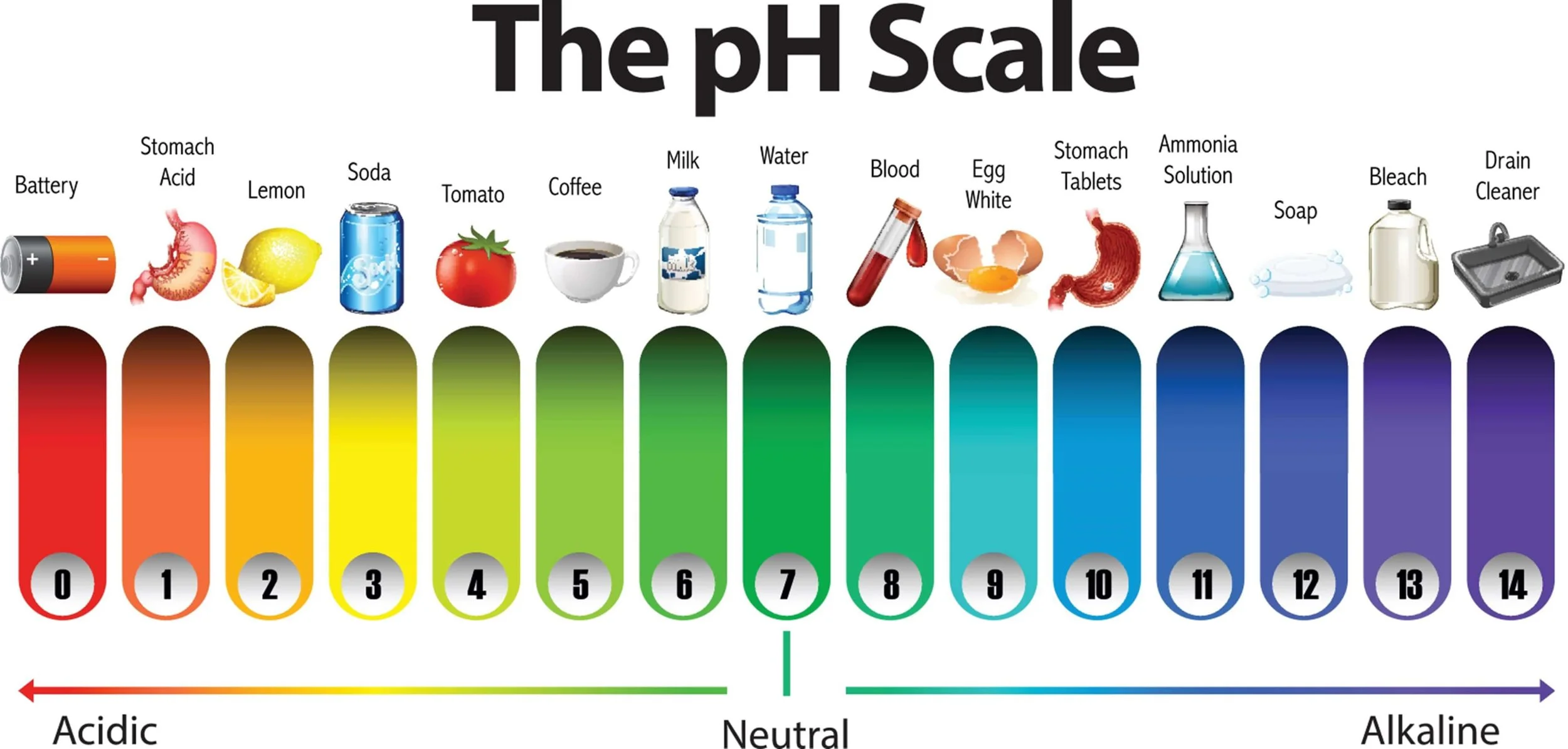
pH is a term used by scientists to quantify the concentration of free hydrogen ions (H+) in a solution. The term pH originates from the French “pouvoir hydrogene”, which translates into “hydrogen power”.
Low pH solutions, aka acids, have many free (excess) hydrogen ions in the solution, whereas high pH solutions have very few hydrogen ions. Acidic solutions have a pH range between 0 to 6.9, with alkaline high pH solutions having a pH between 7.1 to 14. Liquids at pH 7.0 or thereabouts have neutral pH, meaning that the molar concentrations of hydrogen ions (H+) and hydroxyl OH- are equal in solution. The closer the pH of a liquid is to 0, the more acidic it is, with solutions with a pH closer to 14 being stronger alkaline solutions.
The numbers used on the pH scale are inversely related to the concentration of hydrogen ions in the solution. This may sound counterintuitive, but the lower the pH (number), the higher the concentration of hydrogen ions in the liquid and the more acidic the fluid is.
Another important property of pH is the scale used to measure it. The scale is a log scale. A log scale in this context means a difference between any one pH unit. For example, from pH5 to pH4, the pH contains ten times more hydrogen ions in the solution. A change of two pH units, for instance, pH3 to pH1, has a hundred times the concentration of hydrogen ions in the solution.
The effect of pH at the molecular scale on our skin and hair?
pH has two critical effects on biological molecules (discussed below). Both effects can cause significant physical and structural changes to our skin and hair – changes, where pH is either too high or low, can cause irritation, or damage, in some cases, burns. The pH of the products we put on our hair is thus essential to know.
1). High concentrations of hydrogen ions, when in the acidic range, can easily interfere with, break apart (hydrolysis) or denature proteins, lipids and other organic molecules that make up our hair, skin and bodies.
Peptide bonds are the chemical bonds (amine bonds) that hold or link individual amino acids together, giving rise to complex protein structures, including proteins such as Keratin which makes up our skin, nails and hair. Hydrogen ions at sufficient concentrations are known to attack peptide bonds within proteins. This attack can result in proteins degrading and losing their ability to fulfil their function in our skin or hair. Protein also makes up a large proportion of our bodies, so everyone should care about the pH of the products they come into contact with.
As an Amazon Associate, I earn from qualifying purchases - Click here to read our Affiliate Disclaimer
Head and Shoulders conditioners are one of the best on the market (in our opinion), having been in development for many decades. Head and Shoulders shampoos are also very effective hair cleansers. Many of the products within their ranges are now formulated to include excellent hair oils possessing proven hair health and beauty benefits, such as argan and coconut oil. The pH of these particular shampoos and conditioners was tested and found to be pH5.5 and pH4.1, respectively. Give them a try, and let us know how you get on.
2). Hydrogen ions, when at high concentrations, bind to specific sites (called protonation) on the side chains of some amino acids. The binding of these ions results in changes to local and net charges and hydrophobicity. The opposite effect can also occur. In an alkaline environment, protons (hydrogen ions) can be removed (deprotonated) from a protein resulting in a net negative charge, which again affects our hair and skin on both the molecular and large scales.
Hydrophobicity measures how much a molecule, protein, lipid etc., associates (or dislikes) water. “Hydro refers to water, and phobic refers to dislike – hence the conjugate word hydrophobic”.
Both of these pH-medicated effects can quickly enhance the breakup or denaturing of the proteins and lipids of living things. In the case of hair, extreme changes in pH can result in significant cuticle and cortex damage, increasing hair porosity, breakage, and frizz. The effects on our skin can be severe, ranging from burns and scarring to minor skin irritation. Fortunately, haircare and skin contact products are pH formulated to ensure that they do not adversely cause harm.
Why the pH of products is vital to your skin and hair?
pH is essential to both your skin and hair because if incorrect, your hair can suffer from increased damage, or skin and eye irritation can occur. The best-case scenario is a product pH that enhances the product’s intended function or activity whilst having negligible effects on other parts of the body or the environment.
What is the pH of hair?
Hair has a natural pH of 3.67, which is in the acidic range of the pH scale (Ref: D’Souza and Rathi 2015). pH3.67 represents the isoelectric point of hair which means that at this pH, hair possesses an equal balance of charges on its surface, resulting in a net neutral charge. This surface charge increases with increasing pH. When hair experiences pHs higher than pH3.67, such as pH4 or more, hair will gain a net negative charge. The natural or isoelectric pH point of hair can change following hair treatments. For example, the isoelectric point hair becomes more acidic following some hair colouring treatments. The isoelectric point of hair is an essential determinant in haircare product formulation (discussed in more detail below).
What is the pH of the scalp?
The natural pH of the scalp is pH5.5. This pH puts the pH of the scalp in the slightly acidic range (Ref: D’Souza and Rathi 2015). Haircare products with a pH lower than pH5.5 are known to cause scalp irritation (Ref: Gavazzoni Dias, de Almeida et al. 2014).
As an Amazon Associate, I earn from qualifying purchases - Click here to read our Affiliate Disclaimer
These are two paraben and sulfate-free shampoos we have used ourselves and would recommend to everyone looking to experiment with paraben-free and sulfate-free shampoos. They both contain good hair oils, either coconut or argan oil, making them a very healthy and welcome addition to your hair regime. All OGX branded shampoos also have a slightly thicker luxury silky feel making them easier to apply and, in our opinion, a beautiful, somewhat stronger coconutty floral scent. The pH of both these shampoos was tested and found to be pH4.6. Both are definitely worth a go! Let us know how you get on!
What are the physical and structural effects of pH on our hair?
pH has a substantial impact on hair. This is reflected in the pH of the products we put on our hair. For example, strongly alkaline products containing chemicals such as sodium hydroxide (sodium hydroxide has a pH between pH13 -pH14) found in hair colouring and chemical straightening products loosen and open the cuticle. Opening the cuticle is required when hair colouring or chemical straightening to let in the active chemistries, accelerating the process (Ref: Faria., 2013). Sodium hydroxide is known to enhance the protein loss of hair significantly. Hair scientists use protein loss to measure the rate of hair degradation following any particular treatment and are a measure of total hair damage.
Graph adapted from (Franca-Stefoni, Dario et al. 2015) showing the effect of two different hydroxide solutions on hair damage using the protein loss assay.
HairKnowHow has found that the “normal” protein loss range for untreated hair is typically less than 50µg/ml when analysing 0.2g of hair. However, following hair processing with sodium hydroxide, this increases to over 1.0mg/ml, an increase of 20X. This result shows that sodium hydroxide is very damaging to hair, damage that is not repairable.
Research published in the literature has found similar results for example (Franca-Stefoni, Dario et al. 2015) found that sodium hydroxide treatment caused a 276% increase in protein loss and vs 188% increase for guanidine hydroxide. Guanidine hydroxide is a slightly weaker but still powerful hydroxide solution compared to sodium hydroxide. The damage caused by these chemicals can depend on other factors such as the amount of time hair is exposed to them and any pretreatment with oils such as coconut or argan oil.
Sodium hydroxide and Lye relaxers – hair straightening
Sodium hydroxide and the alkaline conditions created by it relax hair permanently. Lye relaxers specifically contain sodium hydroxide. Sodium hydroxide permanently disrupts or breaks the disulfide bonds within keratin molecules by removing a sulfur atom and creating a lanthionine bond. Depending on your hair type, different concentrations of sodium hydroxide are used. The thicker your hair strands, the greater the concentration of sodium hydroxide because they are more resistant hair types. A concentration between 1.85-2.00 % is used on fine hair; for medium hair, 2.06-2.20%; and for coarse hair, a concentration of 2.25-2.40% is commonly used.
Guanidine hydroxide and No-Lye relaxers – hair straightening
No-Lye hair relaxers work similarly to sodium hydroxide by disrupting the disulfide bonds that interlink the keratin molecules. Guanidine hydroxide is slightly milder than sodium hydroxide, which makes it a good alternative. However, guanidine hydroxide still causes a massive amount of permanent hair damage. The downside of using guanidine hydroxide compared to sodium hydroxide is that it needs to be made fresh and used on the same day. Because guanidine hydroxide quickly breaks down and becomes less active over time. Guanidine hydroxide of No-Lye straightening products is made by reacting calcium hydroxide (typically component A of the kit) with guanidine carbonate (component B). This reaction between the two ingredients yields guanidine hydroxide, which is applied to hair immediately.
As with Lye-relaxers (containing sodium hydroxide), different strengths of the No-Lye product are used depending on your hair type. “Normal” strength No-Lye relaxers are targeted at those with fine or medium hair, with “resistant” used for those with coarse hair.
Alkaline pH and its effect on hair
The priority in high street haircare products is always going to what is best for the consumer’s comfort rather than what is best for their hair. This is because hair is essentially an inert material that we cannot “feel” however our skin and eyes do quickly respond to harsher chemistries, causing discomfort. At alkaline pH (pH7-14), there is an increase in the negative electrical charge across a hair’s surface. This negative charge increases frictional forces between individual fibres making hair knotty and increasing cuticle damage and breakage during brushing or washing. Lower pH (<pH5) shampoos and conditioners help to reduce frizz by subsequently lowering the static charge of hair (Ref: Gavazzoni Dias, de Almeida et al. 2014). The complicating factor is that what may be a “better” pH for our hair can be uncomfortable for our eyes. For this reason, many manufacturers have opted for shampoo and conditioner formulations around the pH7 (neutral range) because this is more gentle and less irritating for our eyes.
Alkaline pH its effect on hair hydration and hygral fatigue
Another impact of alkaline pH on hair is that it increases the rate water enters the hair (Ref: Gavazzoni Dias, de Almeida et al. 2014). Water enters the hair due to the cuticle layer's opening on each hair fibre's surface. The ingress of water into hair fibres has many adverse effects, including:
Water breaks (although temporarily) many of the hydrogen bonds present within the hair, which reduces the force required to break the fibre
Water decreases hair’s elasticity (Young’s modulus), resulting in permanently stretched hair fibres if brushed or pulled about excessively. Stretched hair is permanently weaker with often displaced cuticle more prone to breakage and frizz
The opening of the cuticle increases fibre-to-fibre friction and adds to the risk that the edges of individual cuticle cells are damaged; this is because they stick out from the hairs surface
pH in haircare
pH varies within different consumer products. The chosen pH of a consumer product formulation depends on multiple factors. These factors include; where on our body the product will be used, for how long and its intended purposes, known biochemical or physiological responses and proximity of the target location to our eyes or other sensitive areas etc.
What is the pH of shampoo?
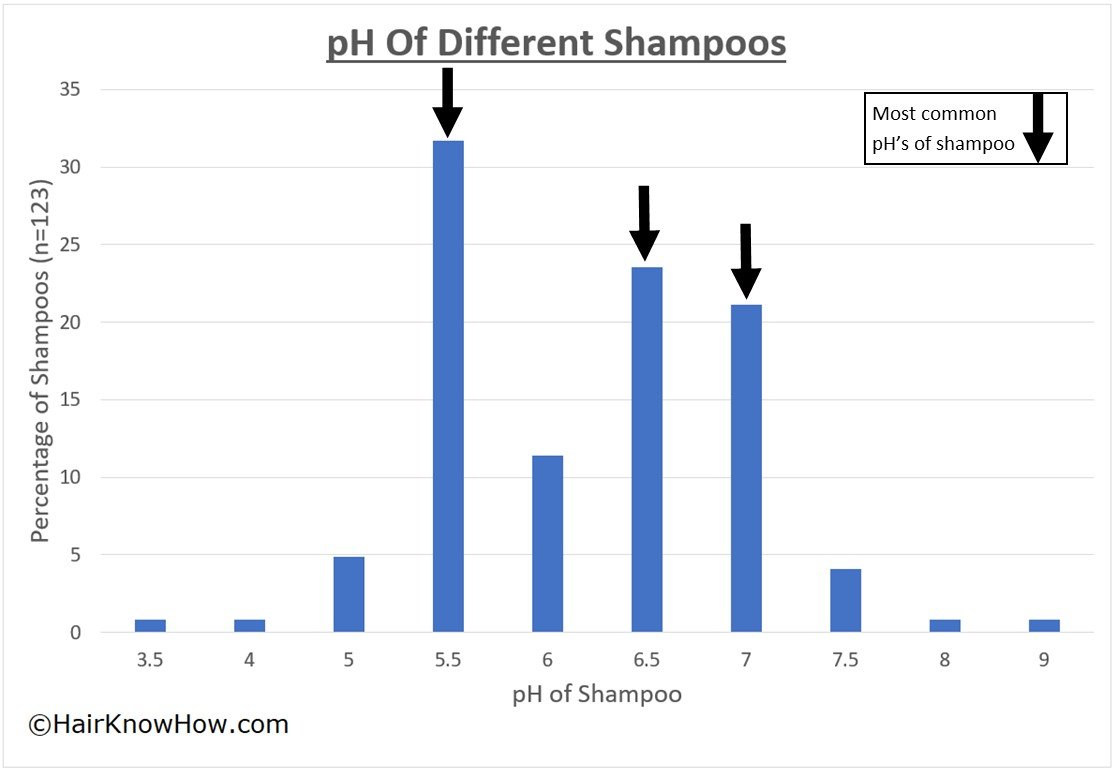
The pH of different haircare products varies widely. Typically, the pH of shampoo will range from pH 3.0 (acidic) to 9.0 (alkaline). The pH of shampoo will depend on the product’s intended purpose (specialist, professional, or highstreet purchasable), the age of its intended consumer, and whether it is marketed as “no more tears” or mild etc.
A small study was carried out on the pH of shampoo products within three key product categories: consumer, professional and specialist shampoos. 123 shampoos were tested in the study, concluding that: 38.2% had a pH lower (more acidic) than 5.5, with 62.8% possessing a pH higher than pH5.5. All children’s shampoos had a pH higher than pH5.5 (Ref: Gavazzoni Dias, de Almeida et al. 2014).
What was observed when breaking down the data into commercial (consumer purchasable in shops) vs professional (used within salons) was that 75% of professional shampoos possess a pH of lower (more acidic) than pH5.5, putting them within the optimal pH range. However, only eight professional shampoos were analysed (Ref: Gavazzoni Dias, de Almeida et al. 2014).
What is the optimal pH of shampoo and conditioner for your hair?
The optimal for a haircare product is between pH 5.5 to 3.5. Ideally, closer to 3.5. This pH is ideal for several interrelated reasons: 1). At pH3.5, hair will possess a neutral charge, which reduces fibre-to-fibre friction. 2). Cuticle cells on the hairs surface do not become raised at this pH, reducing cuticle loss. 3). When the cuticle is closed, water ingress reduces, limiting hygral fatigue. 4). Less water defusing into hair fibres keeps hair more robust and reduces the risk of breakage.
How is pH maintained within a haircare product?
Maintaining the pH within a solution is very important for any haircare consumer product. Shampoo or conditioner may have a pH measured within the factory of pH6.5, but pH can drift up or down over time whilst on the shelf. Changing pH outside a product’s design specification can inactivate the active chemistries within the product or potentially harm the consumer when used. Changes in pH occur naturally (due to temperature etc.), but this can be minimised and controlled using a chemical buffering system. Buffering systems have been used within consumer contact products and laboratories for decades and are essential to product formulation. Buffer systems are chemicals added to a solution that resists the change in pH (discussed below).
Two critical components for an effective buffer system are required: a proton donor and a proton acceptor, aka a weak acid and its conjugate base. These chemicals are explicitly chosen to operate (or buffer) within a specific pH range, usually within 1pH of their pKa. For example, if working at around pH4 citric acid is commonly used.
As an Amazon Associate, I earn from qualifying purchases - Click here to read our Affiliate Disclaimer
We have used these sulfate and silicone-free shampoos and conditioners ourselves and recommend them to everyone looking to experiment with sulfate and silicone-free shampoos. All Faith-in-nature shampoos and conditioners are sulfate and silicone-free. Many products under their brand contain essential hair oils such as coconut oil, making them a very healthy and welcome addition to your hair regime. The Faith-In-Nature products we have tested have possessed, in our opinion, a very soft fragrance. The pH of the shampoo and conditioner above was tested and found to be pH4.9 and pH5.2 respectively. Both are worth trying if you want to move away from traditional shampoo and conditioning products towards more natural-mild ingredients. Let us know how you get on!
The buffering chemistry used within the consumer product depends on three key factors:
Biocompatibility
pKa
Chemical compatibility with other ingredients
Amphoteric surfactants are one common group of ingredients in shampoos affected by changes in pH. Amphoteric surfactants possess a hydrophilic charged moiety that becomes either protonated or deprotonated depending on the pH of their environment. These changes in protonation make them either cationic (positively charged) or anionic (negatively charged), affecting their activity within the product. (Ref: Gavazzoni Dias 2015).
How does temperature affect the pH of the products you use?
Temperature affects pH. When the temperature of a solution increases, the pH decreases – meaning that it becomes slightly more acidic. This is true for all liquids, including haircare and skin products. The opposite is also true, whereby temperature decreases increase the pH of a product making it more alkaline.
Heat on the molecular level is akin to kinetic (movement or vibrational) energy. This is because heat, as we feel it, is essentially little molecular vibrations. The hotter an object or solution is, the more energetic these vibrations are. As the temperature of a haircare product increases, the energy of the molecule vibrations increases (the product feels warmer on our skin). Increases in these molecular vibrations knock off hydrogen ions from product ingredients; this increases the concentration of free hydrogen ions in the solution, decreasing pH, making the solution product more acidic.
These changes in pH are relatively small, typically smaller than 1pH unit per 50 degrees Celsius or 106 Fahrenheit. Also, even the relatively small changes in product pH caused by changing temperature can be lessened or buffered by using a buffer system. Buffer systems are chemicals that absorb (deprotonate) or release (protonate) hydrogen ions at a given pH that stabilise the pH of a product or solution. This is discussed above in more detail.
So should you worry about the temperature of your hair products? The short answer is no. Over the sorts of temperature ranges, hair products are used, there is little change in pH, so there is no need to worry.
If you have any questions or comments about pH and haircare, please get in touch with Dr Palmer or the HairKnowHow team, and we’ll be happy to help.
If you would like to know what would be the best hair care products and ingredients for your hair, then please take a look at our range of hair analyses. Each comes complete with customised hair products and grooming recommendations based on your individual hair results.