Updated January 2023 By Dr Jonathan Palmer
What Are Sulfates Doing To My Hair?
Use this information to help you make an informed choice about whether you want to use sulfate-containing or sulfate-free shampoos.
OK, let's start with the basics…
Which hair care and skin products contain sulfates?
Sulfates, aka sodium lauryl sulfate and sodium laureth sulfate, are widespread ingredients found in many consumer products from shampoos, hand sanitisers, bubble baths, shower gels, and so many more. Sulfates can be found in almost every personal care product that produces bubbles (or lather), making a persistent foam when shaken. Typically, this foam will last for several minutes. Sulfate-free shampoos and bath products usually do not form a persistent foam. If sulfates are present within a product, they will be listed on the ingredient label commonly found on the back of your product. Listing all ingredients is a legal requirement in most jurisdictions around the world.
Example of shampoo and bath products we found that contained sulfate
What is the role of sulfates, and why are they in so many products?
Sulfates are included within personal products because of their proven ability and high efficiency in removing dirt, grease, and oils from our skin, hair and other surfaces we want to clean. These greasy, oily substances could be from either artificial or natural sources – such as sebum, a natural oil found on our skin and hair made by our bodies. Sebum is vital for our skin and hair health, but if left to build up, it can cause skin and hair to feel dirty and greasy. Sulfate-containing shampoos and other bath products efficiently remove sebum.
Typically, within a consumer product, sulfate concentration will vary between 0.01% to 50% depending on the manufacturer and its application. Sulfates have been used for decades in many popular consumer products, and although some people develop sensitivity, reporting dry skin and hair, sulfates are considered safe by every health agency.
What are sulfates doing to your hair?
Sulfates are highly efficient cleaners of your hair, perhaps too good. Sulfates quickly remove dirt, oil and grease from the surface and interior of your hair, which is then easily rinsed off with warm water. Many of the natural oils present within our hair and nutrients we add during conditioning are essential for your hair's health. Sulfates can quickly strip these important substances from your hair, resulting in dryness, especially at the ends, increasing your hair's porosity.
Within the available scientific literature, sulfates (SLES) have been observed accelerating the degradation of the keratin structures within hair, contributing to increased hair porosity. The hypothesis was that this was caused by the dissolving or solubilising of some of the proteins within the hair's cortex. Sulfates are also known to increase natural colour fade, creating a washed-out effect. The research paper in question also showed that coconut oil did impart some protection to the hair fibres being tested.
As an Amazon Associate I earn from qualifying purchases - Click here to read our Affiliate Disclaimer
These are two sulfate-free shampoos we have used ourselves and would recommend to everyone looking to experiment with sulfate-free shampoos. They both contain good hair oils, either coconut or argan oil, making them a very healthy and welcome addition to your hair regime. All OGX branded shampoos also have a slightly thicker luxury silky feel making them easier to apply and, in our opinion, a beautiful, somewhat stronger coconutty floral scent. Definitely worth a go! Let us know how you get on!
A good conditioner helps make hair easier to manage, brush and feel soft, and look shiny with the benefits of being able to be felt for many days after application. You may have noticed that during and after using a sulfate-containing shampoo, it can be difficult to run your fingers through your hair. Sulfates significantly increase the electrical charge of your hair, which in turn increases both frizz and friction. This increase in electrical charge is one of the main reasons for this. Good conditioners contain anti-static and lubricating chemistries to remove the unwanted electrical charge. These include polymers, oils, waxes, hydrolysed amino acids and cationic molecules.
Important: After using a sulfate-containing shampoo, you should thoroughly condition your hair. This essentially means applying a good conditioner and leaving it on for approximately 5 minutes to allow the oils and nutrients to enter your hair fibres. Allowing 5 minutes for your conditioner "to work" can help offset some of the stripping your hair has experienced when using sulfate-containing shampoos.
Should you avoid sulfate-containing shampoos?
If you suffer from a dry scalp, dry hair, dry skin, eye and skin irritation, you should probably find a mild shampoo substitute. Alternatively, try to use your shampoo for less time during washing and make sure you are using a good conditioner - one that ideally contains oils such as coconut or argan oil. These oils can help replace the natural oils removed during shampooing.
It is worth knowing that mild sulfate-free shampoo alternatives can leave your hair feeling greasier and dirtier. You could increase the amount of time you are shampooing if you are experiencing this. This finding is perhaps more important if you have hair that is naturally prone to increased greasiness, for example, Type 1A hair. Experimentation is the key when trying new products. The cleaning efficiency of sulfate-free shampoos is also generally more sensitive (i.e. not as good) to both hard water and different pHs, so your results may vary depending on where you live.
Sulfate-free shampoos are recommended if you colour treat your hair. Colour treatments typically use peroxides and hydroxides that not only cause severe damage to your hair but also strip it of oils and nutrients. So, in this case, it's best to take the mild shampoo option and, as always, use a good conditioner. 😊
Wash your hair, and then rinse the product off. If you don't have sensitive skin or dry hair and are just wanting to wash your hair or body, then sulfate-containing products are fine. But try not to leave them on for too long, as they will thoroughly strip your hair of oil and nutrients – which in turn can lead to dryness, increased porosity, breakage and split ends.
The most important piece of advice we could offer you in all cases is to remember to thoroughly condition your hair with a good conditioner after using any shampoo. Conditioning makes the most significant difference to your hair's health and beauty in most instances.
Sulfate-containing shampoos are not used in isolation. So if you include a good conditioner and deep conditioning regimes into your routine, you should have healthy and clean hair.
Ultimately the choice is yours.
How to tell if your shampoo, shower gel, or handwash contains sulfate detergents?
Firstly, check the label on the product. It is a legal requirement that all ingredients that make up a consumer product be listed. Check whether chemicals such as sodium lauryl sulfate or sodium laureth sulfate are listed. Other common sulfate-containing detergents include ammonium laureth sulfate and ammonium lauryl sulfate.
Secondly, most sulfate-free products make poor foams or lathers. So, for example, if your haircare product forms an intense and persistent (lasting more than a minute) foam, it likely contains a sulfate-containing detergent.
Example of sulfates listed on some typical shampoos and baby products
Also, sulfate can be written as sulphate interchangeably depending on where you live. So keep a lookout if it is spelt differently in your part of the world.
Are sulfates found in conditioners?
No, conditioners do not typically contain sulfates. Sulfates interfere with the conditioning properties of conditioners. However, sulfate is found in many 2-in-1 shampoo and conditioning products. Sulfates are ordinarily absent from conditioners because they usually are used to clean hair before adding a conditioner.
Do sulfates cause hair loss?
No, sulfates do not appear to contribute to hair loss. This myth seems to circulate due to a misinterpretation of a scientific paper. Two scientific reports concluded that sulfates could be deposited within the hair follicle on the root sheath, but no conclusions were drawn regarding hair loss. Yes, the hair follicle is responsible for making and synthesising our hair, but just because a chemical like a sulfate is detected within the follicle after washing does not mean it will interfere with hair growth or the follicle cycle.
No current scientific papers show a causative relationship between sulfates and hair loss. Also, given the widespread and long-term use of sulfate-containing haircare products and elsewhere, any increased risk of hair loss using these chemistries seems highly unlikely.
What are the milder shampoo ingredient alternatives to sulfate-containing detergents?
Alternatives ingredients to sulfate detergents include laureth-5 carboxylic acid, sodium lauryl glucose carboxylate, sodium lauroyl glutamate, sodium lauroyl sarcosinate, sodium methyl cocoyl taurate, sodium lauryl sulfoacetate, disodium lauryl sulfosuccinate, sodium lauroyl isethionate, sodium lauroyl methyl isethionate, cocoglucoside and lauryl glucoside.
Sorry for the list. One or more of these sulfate-free shampoo ingredients can be found in mild shampoos, including some baby shampoo formulations. This is not an exhaustive list but will give those who want to do your own research an excellent place to start examining shampoo bottle ingredients.
What are sulfates?
Sulfates are part of a class of chemical molecules called a surfactant (or lipid). Surfactants occur naturally throughout nature but can also be made artificially within laboratories. Sulfates are powerful cleaning agents, one of the best known with relatively good biocompatibility – this means they clean very well, cause minor skin irritation in people and are safe to use.
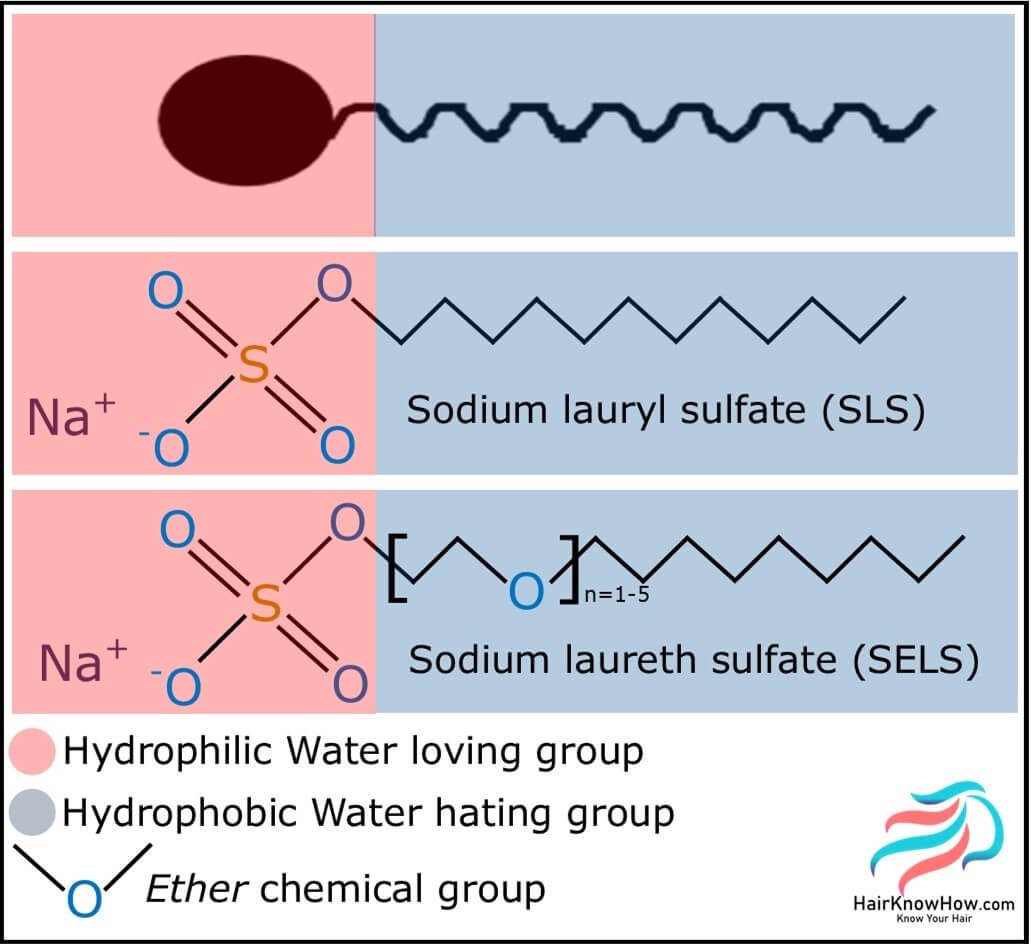
Sulfates used in haircare are classified as either alkyl or alkyl ether sulfates. The alkyl chemistries are sodium (or ammonium) lauryl sulfates, and the ether chemistries are sodium (or ammonium) laureth sulfates.
What do sulfates look like chemically?
Surfactants and lipids are amphipathic molecules which means that they possess both a hydrophobic (water-hating) and hydrophilic (water-loving) chemical group.
A little fact for you: lauryl is an old-fashioned word for dodecyl which itself means 12; this refers to the average carbon chain length of sodium lauryl sulfate!
The super cool thing about surfactants and lipids like sodium lauryl sulfate and sodium laureth sulfate is that at a particular concentration (and at greater concentrations), they start to self-organise and form structures like bubbles, biological membranes, and micelles. Yes, bubbles mixtures that many children play with are the same phenomena. The concentration at which these molecules form bubbles is called the Critical Micelle Concentration (CMC). The CMC is different for each chemical surfactant molecule and can depend on what other chemicals are in solution, temperature and other factors.
A diagram of surfactants and detergents self-organising to make micelles and bubbles
The fact that sulfates, like all detergents, form these bubbles is thought to be an essential property in their cleaning efficacy.



Interesting fact: lipids/detergents are the main components of cellular membranes. Cell membranes are found in every cell of every known life form on Earth. Cells are effectively lipid bubbles – pretty cool stuff. Lipids and surfactants constitute a significant part of your body, and you cannot live without them.
How are sulfates made?
Sodium lauryl sulfate (aka sodium laurilsulfate or sodium dodecyl sulfate) is made by reacting lauryl alcohol with sulfur trioxide, producing hydrogen lauryl sulfate. The hydrogen lauryl sulfate is then neutralised with sodium carbonate, which produces the sodium lauryl sulfate found within our haircare and other products.
As an Amazon Associate I earn from qualifying purchases - Click here to read our Affiliate Disclaimer
These are two sulfate-free shampoos we have used ourselves and would recommend to everyone looking to experiment with sulfate-free shampoos. The OGX and Faith-in-nature shampoos are sulfate-free and contain coconut oil, making them a very healthy and welcome addition to your hair regime. The OGX branded shampoo has a slightly thicker luxury silky feel and, in our opinion, a beautiful, somewhat stronger scent, whereas the Faith-in-nature possesses a much softer fragrance. Both are definitely worth trying. Let us know how you get on!
What are the main challenges faced when developing sulfate-free haircare products?
There remain many challenges that need to be overcome by manufacturers when developing new sulfate-free shampoos. The good news is that even the major producers of shampoos are now getting involved and fronting serious Research and Development (R&D) money and effort into their development. With increased R&D, new patents have been appearing covering novel sulfate-free formulations that will hopefully function and perform to a high standard. This is great for us, the consumer, as this will help make sulfate-free shampoos less expensive and niche and more affordable for the haircare consumer.
Research and development being conducted in a laboratory
Some of these challenges (discussed below) may not seem important, especially to us, the consumer, and many are a bit technical. Still, it is good to know a little about what goes into making or formulating a shampoo, along with some of the R&D and cost considerations manufacturers face, before us, the consumer, get to try them.
The cost of new and exotic surfactants is more expensive than "standard" sulfate chemistries. This additional cost can be due to difficulties in manufacturing the novel surfactant and their initial scarcity. Such challenges can be overcome with economies of scale and optimisations of the methods used in their synthesis. This may sound easy, but it can be tricky given there is rightly a desire to avoid the use of toxic chemicals in the process that must be removed and tested before final product formulation.
When more of any ingredient is added to a product, that product inevitably becomes more expensive. The concentration (or amount) of replacement surfactant in sulfate-free formulations typically needs to be higher than sulfate-based chemistries. This is to achieve adequate foaming and cleaning performance. This is driven partly by the consumer that has preconformed expectations of foaming, thickness (or viscosity) and cleaning qualities based on what we have all been accustomed to, having used sulfate-containing products over many years. After all, we typically want lots of bubbles (the more, the better), the product to be thick and not watery, which then runs off our hands or hair when using it. Plus, we want it to do a good job cleaning our hair.
Simple and safe household ingredients like salt (sodium chloride) can thicken (increase the viscosity) sulfate detergents. Many alternative detergents do not thicken following salt addition, so other chemicals are needed. These different chemistries may then interfere with other common additives like silicones found within shampoos – decreasing the performance of the shampoo. This goes back to the usability of the product (it not being too runny) and the consumer's user expectation.
The complex interactions between new novel sulfate-free detergents with the other ingredients are not necessarily known, which can make designing, developing and testing a product much longer and more expensive. It is often unknown how these new detergents or co-ingredients interact and behave with our hair and skin on their own or with their co-ingredients. This knowledge is essential as we all hope that these milder shampoo alternatives are kinder to our hair and skin. Still, this finding often results from a massive amount of R&D before product release and following consumer testing within the home environment. It can take many years for shampoo performance and the long-term impact of these new detergents on our hair and skin to be known. Sometimes it's better the devil, you know.
We hope you found this exciting and informative if you have any questions or want us to cover a specific hair topic, please let us know.
Give HairKnowHow a try. Check out our shop using the link, or contact us if you have any questions about sulfate containing shampoos or any other hair topics.
The Hairknowhow team.